Tetralogy of Fallot
| Tetralogy of Fallot | |
|---|---|
| Classification and external resources | |
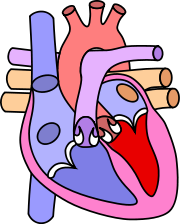
 Diagram of a healthy heart and one suffering from tetralogy of Fallot |
|
| ICD-10 | Q21.3 |
| ICD-9 | 745.2 |
| OMIM | 187500 |
| DiseasesDB | 4660 |
| MedlinePlus | 001567 |
| eMedicine | emerg/575 |
| MeSH | D013771 |
Tetralogy of Fallot (TOF) is a congenital heart defect which is classically understood to involve four anatomical abnormalities (although only three of them are always present). It is the most common cyanotic heart defect, and the most common cause of blue baby syndrome. [1]
It was described in 1672 by Niels Stensen, in 1773 by Edward Sandifort, and in 1888 by the French physician Étienne-Louis Arthur Fallot, for whom it is named.[2]
Contents |
Anatomic morphology
Primary four malformations
"Tetralogy" denotes a four-part phenomenon in various fields, including literature, and the four parts the syndrome's name implies are its four signs. This is not to be confused with the similarly named teratology, a field of medicine concerned with abnormal development and congenital malformations, which thereby includes tetralogy of Fallot as part of its subject matter.
As such, by definition, tetralogy of Fallot involves exactly four heart malformations which present together:
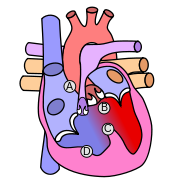
| Condition | Description |
|---|---|
| A: Pulmonary stenosis | A narrowing of the right ventricular outflow tract and can occur at the pulmonary valve (valvular stenosis) or just below the pulmonary valve (infundibular stenosis). Infundibular pulmonic stenosis is mostly caused by overgrowth of the heart muscle wall (hypertrophy of the septoparietal trabeculae),[3] however the events leading to the formation of the overriding aorta are also believed to be a cause. The pulmonic stenosis is the major cause of the malformations, with the other associated malformations acting as compensatory mechanisms to the pulmonic stenosis.[4] The degree of stenosis varies between individuals with TOF, and is the primary determinant of symptoms and severity. This malformation is infrequently described as sub-pulmonary stenosis or subpulmonary obstruction.[5] |
| B: Overriding aorta | An aortic valve with biventricular connection, that is, it is situated above the ventricular septal defect and connected to both the right and the left ventricle. The degree to which the aorta is attached to the right ventricle is referred to as its degree of "override." The aortic root can be displaced toward the front (anteriorly) or directly above the septal defect, but it is always abnormally located to the right of the root of the pulmonary artery. The degree of override is quite variable, with 5-95% of the valve being connected to the right ventricle.[3] |
| C: ventricular septal defect (VSD) | A hole between the two bottom chambers (ventricles) of the heart. The defect is centered around the most superior aspect of the ventricular septum (the outlet septum), and in the majority of cases is single and large. In some cases thickening of the septum (septal hypertrophy) can narrow the margins of the defect.[3] |
| D: Right ventricular hypertrophy | The right ventricle is more muscular than normal, causing a characteristic boot-shaped (coeur-en-sabot) appearance as seen by chest X-ray. Due to the misarrangement of the external ventricular septum, the right ventricular wall increases in size to deal with the increased obstruction to the right outflow tract. This feature is now generally agreed to be a secondary anomaly, as the level of hypertrophy generally increases with age.[6] |
There is anatomic variation between the hearts of individuals with tetralogy of Fallot. Primarily, the degree of right ventricular outflow tract obstruction varies between patients and generally determines clinical symptoms and disease progression.
Additional anomalies
In addition, tetralogy of Fallot may present with other anatomical anomalies, including:
- stenosis of the left pulmonary artery, in 40% of patients
- a bicuspid pulmonary valve, in 40% of patients
- right-sided aortic arch, in 25% of patients
- coronary artery anomalies, in 10% of patients
- a foramen ovale or atrial septal defect, in which case the syndrome is sometimes called a pentalogy of Fallot[7]
- an atrioventricular septal defect
- partially or totally anomalous pulmonary venous return
- forked ribs and scoliosis
Tetralogy of Fallot with pulmonary atresia (pseudotruncus arteriosus) is a severe variant[8] in which there is complete obstruction (atresia) of the right ventricular outflow tract, causing an absence of the pulmonary trunk during embryonic development. In these individuals, blood shunts completely from the right ventricle to the left where it is pumped only through the aorta. The lungs are perfused via extensive collaterals from the systemic arteries, and sometimes also via the ductus arteriosus.
Epidemiology and etiology
Tetralogy of Fallot occurs in approximately 400 per million live births.[9]
Its cause is thought to be due to environmental or genetic factors or a combination. It is associated with chromosome 22 deletions and diGeorge syndrome.
Specific genetic associations include:
It occurs slightly more often in males than in females.
Embryology studies show that it is a result of anterior malalignment of the aorticopulmonary septum, resulting in the clinical combination of a VSD, pulmonary stenosis, and an overriding aorta. Right ventricular hypertrophy results from this combination, which causes resistance to blood flow from the right ventricle.
Pathophysiology and symptoms
Tetralogy of Fallot results in low oxygenation of blood due to the mixing of oxygenated and deoxygenated blood in the left ventricle via the VSD and preferential flow of the mixed blood from both ventricles through the aorta because of the obstruction to flow through the pulmonary valve. This is known as a right-to-left shunt. The primary symptom is low blood oxygen saturation with or without cyanosis from birth or developing in the first year of life. If the baby is not cyanotic then it is sometimes referred to as a "pink tet".[14] Other symptoms include a heart murmur which may range from almost imperceptible to very loud, difficulty in feeding, failure to gain weight, retarded growth and physical development, dyspnea on exertion, clubbing of the fingers and toes, and polycythemia.
Children with tetralogy of Fallot may develop "tet spells". The precise mechanism of these episodes is in doubt, but presumably results from a transient increase in resistance to blood flow to the lungs with increased preferential flow of desaturated blood to the body. Tet spells are characterized by a sudden, marked increase in cyanosis followed by syncope, and may result in hypoxic brain injury and death. Older children will often squat during a tet spell, which increases systemic vascular resistance and allows for a temporary reversal of the shunt.
Diagnosis
The abnormal "coeur-en-sabot" (boot-like) appearance of a heart with tetralogy of Fallot is easily visible via chest x-ray, and before more sophisticated techniques became available, this was the definitive method of diagnosis. Congenital heart defects are now diagnosed with echocardiography, which is quick, involves no radiation, is very specific, and can be done prenatally.
Treatment
Emergency management of tet spells
Prior to corrective surgery, children with tetralogy of Fallot may be prone to consequential acute hypoxia (tet spells), characterized by sudden cyanosis and syncope. These may be treated with beta-blockers such as propranolol, but acute episodes may require rapid intervention with morphine to reduce ventilatory drive and a vasopressor such as epinephrine, phenylephrine, or norepinephrine to increase blood pressure. Oxygen is effective in treating spells because it is a potent pulmonary vasodilator and systemic vasoconstrictor. This allows more blood flow to the lungs. There are also simple procedures such as squatting and the knee chest position which increases aortic wave reflection, increasing pressure on the left side of the heart, decreasing the right to left shunt thus decreasing the amount of deoxygenated blood entering the systemic circulation.[15]
Palliative surgery
The condition was initially thought untreatable until surgeon Alfred Blalock, cardiologist Helen B. Taussig, and lab assistant Vivien Thomas at Johns Hopkins University developed a palliative surgical procedure, which involved forming an anastomosis between the subclavian artery and the pulmonary artery (See movie "Something the Lord Made").[16] It was actually Helen Taussig who convinced Alfred Blalock that the shunt was going to work. This redirected a large portion of the partially oxygenated blood leaving the heart for the body into the lungs, increasing flow through the pulmonary circuit, and greatly relieving symptoms in patients. The first Blalock-Thomas-Taussig shunt surgery was performed on 15-month old Eileen Saxon on November 29, 1944 with dramatic results.
The Potts shunt[17] and the Waterston-Cooley shunt[18][19] are other shunt procedures which were developed for the same purpose. These are no longer used.
Currently, Blalock-Thomas-Taussig shunts are not normally performed on infants with TOF except for severe variants such as TOF with pulmonary atresia (pseudotruncus arteriosus).
Total surgical repair
The Blalock-Thomas-Taussig procedure, initially the only surgical treatment available for Tetralogy of Fallot, was palliative but not curative. The first total repair of Tetralogy of Fallot was done by a team led by C. Walton Lillehei at the University of Minnesota in 1954 on a 11-year-old boy.[20] Successful total repair on infants has had success from 1981, with research indicating that it has a comparatively low mortality rate. [21]
Total repair of Tetralogy of Fallot initially carried a high mortality risk. This risk has gone down steadily over the years. Surgery is now often carried out in infants one year of age or younger with less than 5% perioperative mortality. The open-heart surgery is designed (1) to relieve the right ventricular outflow tract stenosis by careful resection of muscle and (2) to repair the VSD with a Gore-Tex patch or a homograft. Additional reparative or reconstructive surgery may be done on patients as required by their particular cardiac anatomy.
Prognosis
Untreated, tetralogy of Fallot rapidly results in progressive right ventricular hypertrophy due to the increased resistance on the right ventricle. This progresses to heart failure (dilated cardiomyopathy) which begins in the right heart and often leads to left heart failure. Actuarial survival for untreated tetralogy of Fallot is approximately 75% after the first year of life, 60% by four years, 30% by ten years, and 5% by forty years.
Patients who have undergone total surgical repair of tetralogy of Fallot have improved hemodynamics and often have good to excellent cardiac function after the operation with some to no exercise intolerance (New York Heart Association Class I-II). Surgical success and long-term outcome greatly depends on the particular anatomy of the patient and the surgeon's skill and experience with this type of repair.
Ninety percent of patients with total repair as infants develop a progressively leaky pulmonary valve as the heart grows to its adult size but the valve does not. Patients also often have damage to the electrical system of the heart from surgical incisions, causing abnormalities as detected by EKG and/or arrhythmias.
Long-term follow up studies show that patients with total repair of TOF are at risk for sudden cardiac death and for heart failure. Therefore, lifetime follow-up care by an adult congenital cardiologist is recommended to monitor these risks and to recommend treatment, such as interventional procedures or re-operation, if it becomes necessary.
Antibiotic prophylaxis is indicated during dental treatment in order to prevent infective endocarditis. The use of antibiotics is no longer required by cardiologists and varies from case to case.
Notable people born with the defect
- Shaun White,[22] snowboarder and 2006 and 2010 Olympic gold medallist
- Beau Casson,[23] Australian cricketer
- Dennis McEldowney,[24]
- Yu-Yao Teoh
- Rhode Island violinist Darcy Davis
- Renske Maritz
- Samuel Sander, Pianist
See also
- Trilogy of Fallot
References
- ↑ Breitbart R, Fyler, D. Tetralogy of Fallot. In Nadas' Pediatric Cardiology, 2ed, Ed. Keane, Locke, & Fyler, Philadelphia: Saunders-Elsevier, 2006, p. 559.
- ↑ synd/2281 at Who Named It?
- ↑ 3.0 3.1 3.2 Gatzoulis MA, Webb GD, Daubeney PE. (2005) Diagnosis and Management of Adult Congenital Heart Disease. Churchill Livingstone, Philadelphia. ISBN 0443071039.
- ↑ Bartelings M, Gittenberger-de Groot A (1991). "Morphogenetic considerations on congenital malformations of the outflow tract. Part 1: Common arterial trunk and tetralogy of Fallot". Int. J. Cardiol. 32 (2): 213–30. doi:10.1016/0167-5273(91)90329-N. PMID 1917172.
- ↑ Anderson RH, Weinberg. The clinical anatomy of tetralogy of Fallot. Cardiol Young. 2005 15;38-47. PMID 15934690.
- ↑ Anderson RH, Tynan M. Tetralogy of Fallot – a centennial review. Int J Cardiol. 1988 21; 219-232. PMID 3068155.
- ↑ Cheng TO (1995). "Pentalogy of Cantrell vs pentalogy of Fallot". Tex Heart Inst J 22 (1): 111–2. PMID 7787464.
- ↑ Farouk A, Zahka K, Siwik E, et al. (December 2008). "Individualized approach to the surgical treatment of tetralogy of Fallot with pulmonary atresia". Cardiol Young 19 (1): 1–10. doi:10.1017/S1047951108003430. PMID 19079949. http://journals.cambridge.org/abstract_S1047951108003430.
- ↑ Child JS (July 2004). "Fallot's tetralogy and pregnancy: prognostication and prophesy". J. Am. Coll. Cardiol. 44 (1): 181–3. doi:10.1016/j.jacc.2004.04.009. PMID 15234430. http://linkinghub.elsevier.com/retrieve/pii/S0735109704006990.
- ↑ Eldadah ZA, Hamosh A, Biery NJ, et al. (January 2001). "Familial Tetralogy of Fallot caused by mutation in the jagged1 gene". Hum. Mol. Genet. 10 (2): 163–9. doi:10.1093/hmg/10.2.163. PMID 11152664. http://hmg.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11152664.
- ↑ Goldmuntz E, Geiger E, Benson DW (November 2001). "NKX2.5 mutations in patients with tetralogy of fallot". Circulation 104 (21): 2565–8. doi:10.1161/hc4601.098427. PMID 11714651. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11714651.
- ↑ Pizzuti A, Sarkozy A, Newton AL, et al. (November 2003). "Mutations of ZFPM2/FOG2 gene in sporadic cases of tetralogy of Fallot". Hum. Mutat. 22 (5): 372–7. doi:10.1002/humu.10261. PMID 14517948.
- ↑ Lambrechts D, Devriendt K, Driscoll DA, et al. (June 2005). "Low expression VEGF haplotype increases the risk for tetralogy of Fallot: a family based association study". J. Med. Genet. 42 (6): 519–22. doi:10.1136/jmg.2004.026443. PMID 15937089. PMC 1736071. http://jmg.bmj.com/cgi/pmidlookup?view=long&pmid=15937089.
- ↑ "Tetralogy of Fallot: Overview - eMedicine". http://emedicine.medscape.com/article/760387-overview. Retrieved 2009-01-02.
- ↑ Murakami T (2002). "Squatting: the hemodynamic change is induced by enhanced aortic wave reflection". Am. J. Hypertens. 15 (11): 986–8. doi:10.1016/S0895-7061(02)03085-6. PMID 12441219.
- ↑ "First Operations; Blalock - Taussig Shunt". http://www.medicalarchives.jhmi.edu/firstor.htm. Retrieved 2007-11-15.
- ↑ Boshoff D, Budts W, Daenen W, Gewillig M (January 2005). "Transcatheter closure of a Potts' shunt with subsequent surgical repair of tetralogy of fallot". Catheter Cardiovasc Interv 64 (1): 121–3. doi:10.1002/ccd.20247. PMID 15619282.
- ↑ Daehnert I, Wiener M, Kostelka M (May 2005). "Covered stent treatment of right pulmonary artery stenosis and Waterston shunt". Ann. Thorac. Surg. 79 (5): 1754–5. doi:10.1016/j.athoracsur.2003.11.059. PMID 15854971. http://linkinghub.elsevier.com/retrieve/pii/S0003497504002528.
- ↑ "Systemic to Pulmonary Artery Shunting for Palliation: - eMedicine". http://emedicine.medscape.com/article/905950-overview. Retrieved 2009-01-02.
- ↑ Lillehei CW; Cohen, M; Warden, HE; Read, RC; Aust, JB; Dewall, RA; Varco, RL (1955). [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1465089 "Direct Vision Intracardiac Surgical Correction of the Tetralogy of Fallot, Pentalogy of Fallot, and Pulmonary Atresia Defects Report of First Ten Cases"]. Ann Surg. 142 (3): 418–442. doi:10.1097/00000658-195509000-00010. PMID 13249340.
- ↑ "Total correction of tetralogy of fallot in the first year of life: late results". http://ats.ctsnetjournals.org/cgi/content/full/74/1/133..
- ↑ "SI.com - SI Adventure - Double Ripper - Wednesday July 02, 2003 05:14 PM". http://sportsillustrated.cnn.com/features/siadventure/29/boarding/. Retrieved 2009-01-02.
- ↑ "New twist in Casson's amazing journey - Cricket - Sport - smh.com.au". http://www.smh.com.au/news/cricket/new-twist-in-cassons-amazing-journey/2008/06/05/1212259004804.html. Retrieved 2009-01-02.
- ↑ http://www.bookcouncil.org.nz/writers/mceldowney.html
External links
- Tetralogy of Fallot - Children's Hospital Boston
- Diseases and Conditions Index: TOF at National Institute of Health
- Tetralogy of Fallot information from Seattle Children's Hospital Heart Center
- Tetralogy of Fallot Information by the University of Michigan C.S. Mott Children’s Hospital
- Diagram of the condition at University of Utah
- Designer Heart, A Congenital Heart Defect Social Network.
|
||||||||||||||||||||||||||||||||||||